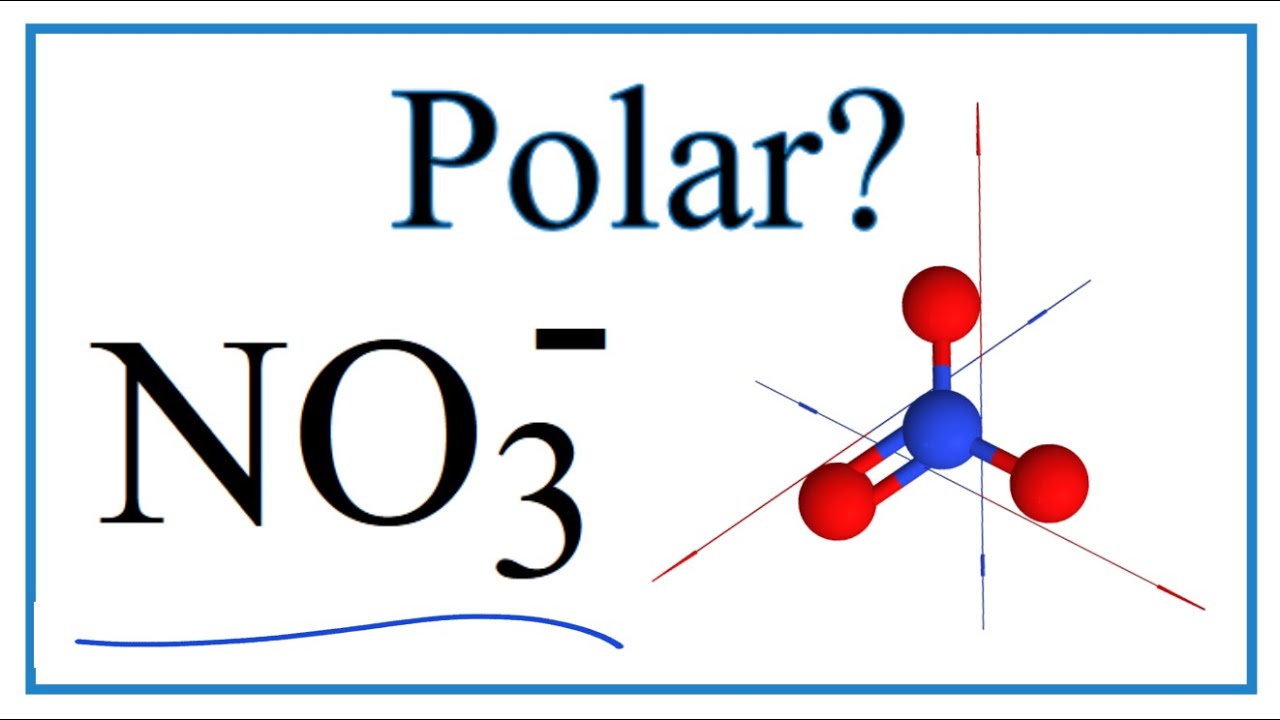
Is NO3 Polar or Nonpolar? (Nitrate ion) YouTube
It is non polar because it is a symmetrical trigonal planar structure. Explanation: I assume you mean NO3- (the nitrate anion); there is no NO3 molecule. It is non-polar because it has a trigonal planar structure and the symmetry means that there is an even distribution of electron charge density over the three N - O bonds.

SOLVED List and know all of the names of the monatomic cations and
Want to know the reason? Let's dive into it! HNO3 is a POLAR molecule because the O-H bond present in the molecule is polar, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire HNO3 molecule polar.
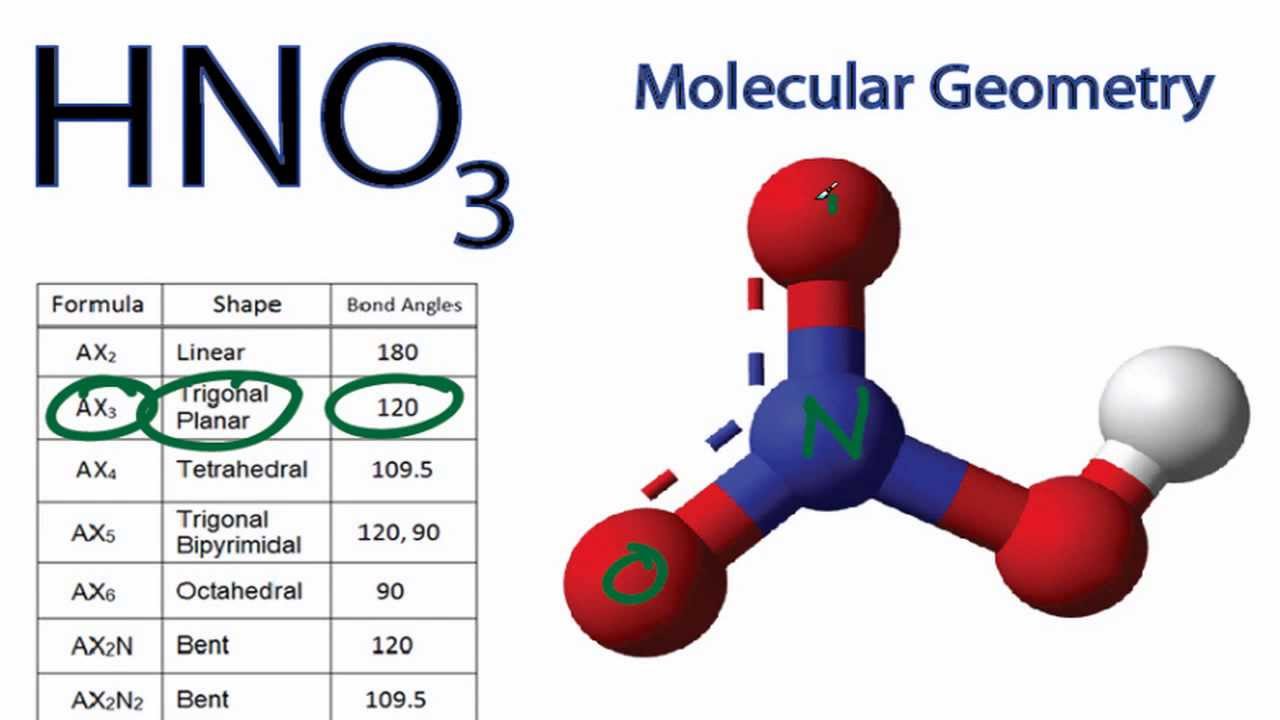
is no3 polar or nonpolar
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself.. {NO3-}\), and \(\ce{NH4+}\), are held together by polar covalent bonds. However, these polyatomic ions.

NO3 Molecular Geometry / Shape and Bond Angles YouTube
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.

Solved Question 9 Match the correct name to the ion or
Is NO3- Polar or Nonpolar? (Nitrate) Geometry of Molecules 2.86K subscribers Subscribe Subscribed 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 Share 664 views 1 year ago Polarity of.

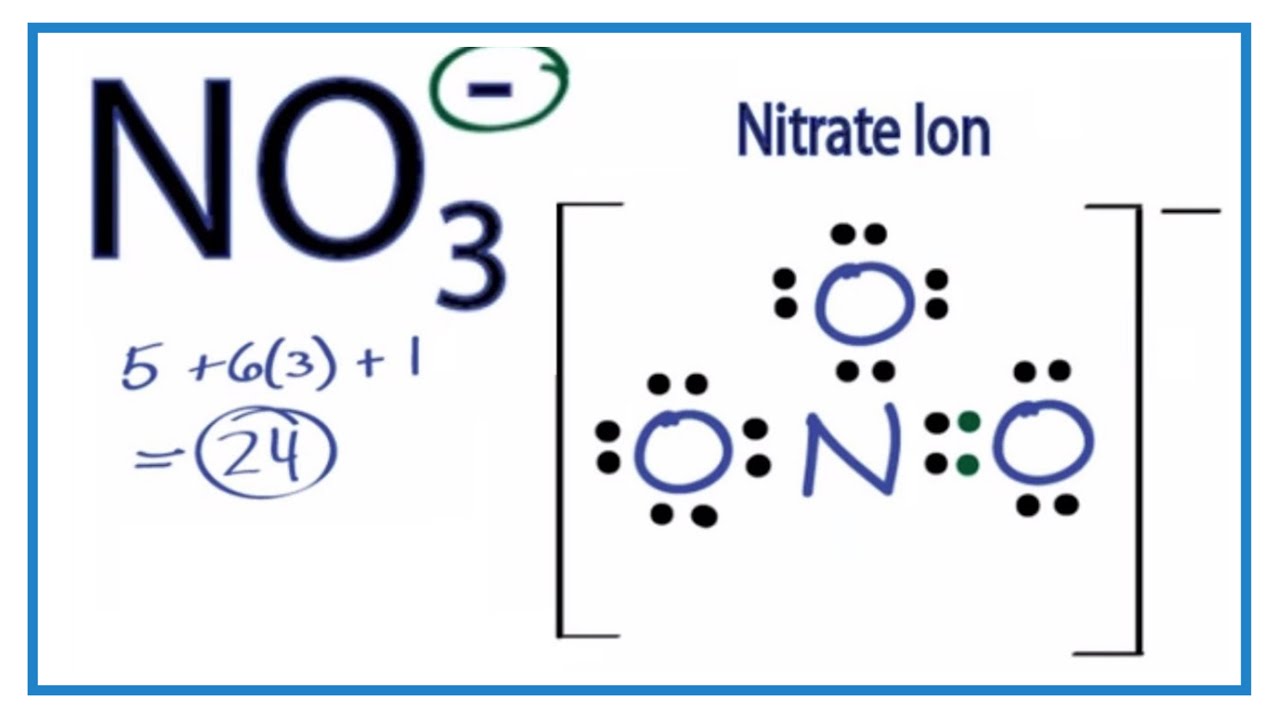
Solved 2. Draw Lewis Structures For CO2, SO, And NO3 3. G...
Unlike polar bonds, non-polar bonds share electrons equally. A bond between two atoms or more atoms is non-polar if the atoms have the same electronegativity or a difference in electronegativities that is less than 0.4. An example of a non-polar bond is the bond in chlorine. Chlorine contains two chlorine atoms.

Is SO3 Polar or Nonpolar? Techiescientist
NO3- (or Nitrate ion) is a NONPOLAR ion because all the three bonds (one N=O and two N-O bonds) are equidistant and NO3- has symmetrical geometry. Let me explain this in detail with the help of NO3- lewis structure and its 3D geometry. Why is NO3- a Nonpolar ion? (Explained in 2 Steps)

is no3 polar or nonpolar
Is NO3- Polar or Nonpolar? (Nitrate ion) Wayne Breslyn 716K subscribers Subscribe Subscribed 15K views 2 years ago Learn to determine if NO3- is polar or nonpolar based on the polarity.
Workouts for weight loss for beginners, no3 shape
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

Is NO3 Polar or Nonpolar? Polarity of Nitrate
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Lewis Structure NO3 plus dipoles, shape, angles, resonance and formal
Nitrate [NO3]- is a non-polar molecular ion. It consists of a nitrogen (N) atom and three oxygen (O) atoms. The nitrogen atom is present at the center of the molecular ion, while three oxygen (O) atoms occupy terminal positions, one on each side, making a symmetrical trigonal planar shape.
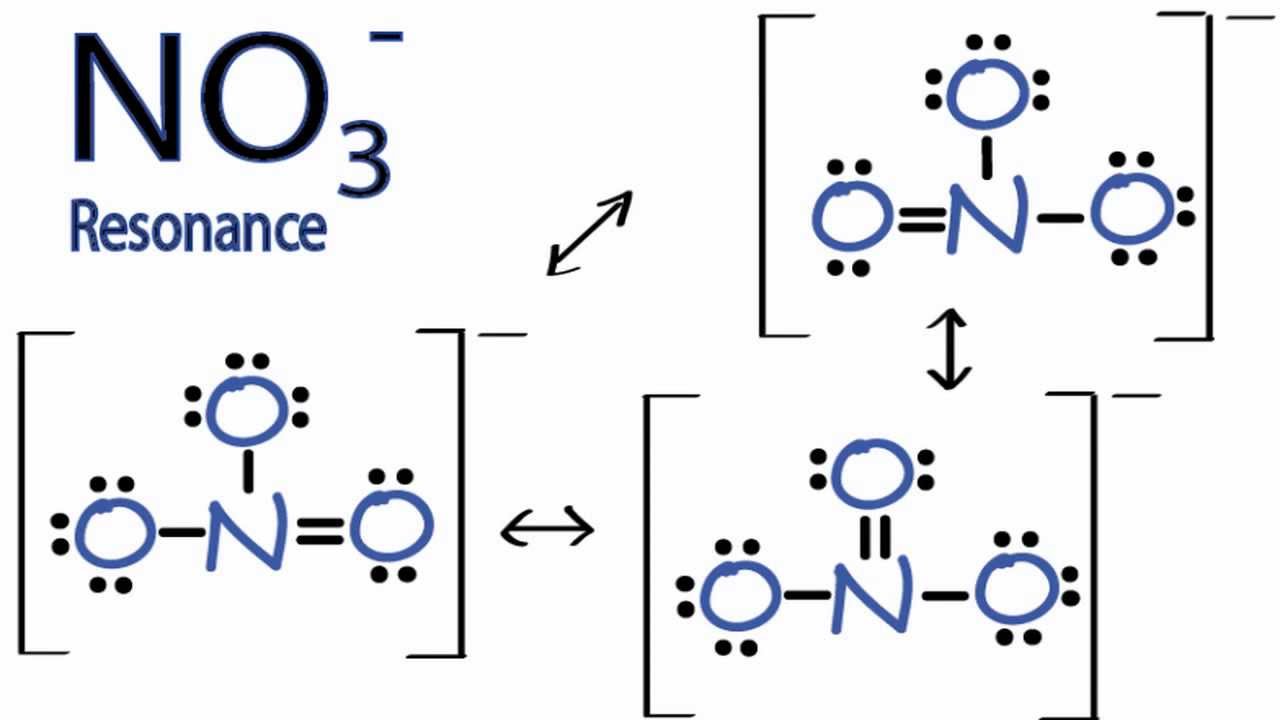
Resonance Structures for NO3 (Nitrate Ion) YouTube
NO3 is a non-polar molecule because of the symmetrical structure irrespective of the presence of a double bond, which cancels out all the dipole moments inside the molecule. As there is no net dipole moment, there will be no separation of charges between the two ends of the molecule and hence no polarity. Contents show

Draw The Lewis Structure Of No3 Fotodtp
A rough approximation of the electronegativity differences associated with covalent, polar covalent, and ionic bonds is shown in Figure 3.5.0.4 3.5.0. 4. This table is just a general guide, however, with many exceptions. For example, the H and F atoms in HF have an electronegativity difference of 1.9, and the N and H atoms in NH 3 a difference.

Is Hexane Polar or Nonpolar
DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-e.Snapchat: https://www.snapchat.com/add/kravonoInstagram: https://www.instagram.c.

Is NO3 Polar or Nonpolar? Techiescientist
NO3- is polar.I just think about it the way chad said for the solubility rules, NO3- are soluble in water and since water is polar and "like dissolves like" then NO3- is polar

Lectura Enlaces covalentes Biología I Association LEA
It is non-polar because it has a trigonal planar structure and the symmetry means that there is an even distribution of electron charge density over the three N−O bond. Whilst often draw the structure of NO3−I as a Lewis structure comparison double and single bonds, the reality is that the three N−O bonds are actually identical.